Fighting the SFTS virus with lipoxygenase: a new hope for treatment
GA, UNITED STATES, July 18, 2025 /EINPresswire.com/ -- A deadly tick-borne illness, severe fever with thrombocytopenia syndrome (SFTS), continues to claim lives with no specific antiviral treatment. However, a breakthrough study reveals that lipoxygenase (LOX), an enzyme previously known for its role in inflammation, etc., can effectively halt the progression of SFTS virus (SFTSV) infection. By disrupting the viral envelope through lipid peroxidation, LOX prevents the virus from infecting host cells, offering a potential new strategy for combating this deadly disease. This discovery could have far-reaching implications for the treatment of other dangerous enveloped viral infections as well.
Severe fever with thrombocytopenia syndrome (SFTS) was reported in Asia, with a fatality rate that can reach as high as 50%. Despite efforts with existing antiviral drugs, no treatment has consistently proven effective. Lipoxygenase (LOX), a natural enzyme involved in the oxidation of fatty acids, has recently attracted attention for its antiviral properties. Research has shown that LOX can alter the structure of viral envelopes via lipid peroxidation, preventing the virus from entering host cells. Given the urgent need for effective therapies, this discovery suggests that LOX might be a key player in the battle against SFTSV and similar viral infections. Based on these challenges, further research is essential to understand how LOX can be leveraged in antiviral treatment.
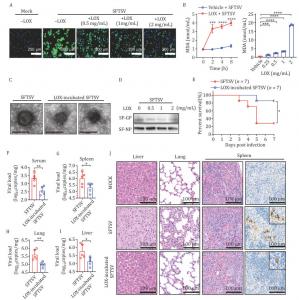
Published (DOI: 10.1093/procel/pwae061) in Protein & Cell (October 2024), a letter-style study reveals the remarkable antiviral potential of lipoxygenase (LOX) in fighting the severe fever with thrombocytopenia syndrome virus (SFTSV). The new research, led by teams from the Academy of Military Medical Science and other esteemed institutions, shows that LOX can disrupt the viral lipid envelope through peroxidation, preventing the virus from infecting host cells. These findings could lead to the development of innovative antiviral therapies, not only for SFTSV but also for other enveloped viruses.
The study demonstrated LOX's powerful ability to prevent SFTSV infection in lab-grown cells. By catalyzing lipid peroxidation, LOX disrupts the viral envelope, which is essential for the virus to bind to and infect host cells. The researchers tested this mechanism in both in vitro experiments and a mouse model, where they observed a significant reduction in viral replication and less severe tissue damage in mice treated with LOX-pre-incubated virus. LOX pre-treatment also showed promise in combating other enveloped viruses, including influenza and vesicular stomatitis virus, highlighting its broad-spectrum antiviral properties. These results suggest that LOX could be a powerful tool for stopping viral infections at multiple stages of the disease.
Dr. Wei Liu, one of the corresponding authors of the study, explains: This research reveals a new potential weapon against viral infections. LOX is capable of destabilizing the viral envelope by catalyzing lipid peroxidation, rendering the virus unable to infect host cells. This discovery is exciting because it not only opens new possibilities for treating SFTSV but also paves the way for future antiviral therapies targeting other dangerous enveloped viruses.
The findings from this study position LOX as a promising candidate for future antiviral therapies. Its ability to disrupt the viral membrane offers a novel approach to treating SFTSV, which has proven difficult to manage with existing drugs. Furthermore, LOX's broad-spectrum activity against other enveloped viruses suggests that it could play a significant role in tackling various viral infections. Future research will be crucial to refine LOX-based treatments, optimize its use in clinical settings, and explore its full potential as a therapeutic tool for viral diseases.
DOI
10.1093/procel/pwae061
Original Source URL
https://doi.org/10.1093/procel/pwae061
Funding information
The study was supported by the National Natural Science Foundation of China (22121003).
Lucy Wang
BioDesign Research
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.